Media
Martin Shkreli Banned From Pharmaceutical Industry, Ordered to Pay $64.6 Million Over Daraprim
On January 14, 2022, United States District Court Judge Denise Cote ordered Martin Shkreli to pay a total of $64.6 million to seven states defrauded by Shkreli’s pharmaceutical company, Vyera, through its illegal pricing scheme for the drug Daraprim. These states include New York, California, Ohio, Pennsylvania, Illinois, North Carolina and Virginia. Judge Cote further ordered an injunction forever banning Shkreli from participating “in any capacity” in the pharmaceutical industry.
Daraprim Pricing Scheme
As covered previously on this blog, Daraprim was first made available in the 1950s and is used primarily for the treatment of toxoplasmosis and malaria. Upon acquiring exclusive rights to Daraprim in 2015, Vyera immediately raised the price of the drug from $17/tablet to $750/tablet, an increase of 4000%. The previous company producing Daraprim had raised prices from $7 per tablet. This resulted in a combined price increase of 11,000% over one-half decade.
|
|
Price |
Percent change from 2010 |
|
Daraprim, 2010 |
$6.75 |
– |
|
Daraprim, 2011-2015 |
$17.60 |
+260% |
|
Daraprim, August 2015 (Vyera) |
$750.00 |
+11,111% |
Federal Trade Commission and Attorneys General’s Antitrust Claims
In the aftermath of the price increase, Vyera immediately faced criticism, public outrage, and eventually congressional hearings. The Federal Trade Commission (FTC) filed an antitrust claim against Vyera and Martin Shkreli, and several states including New York and California joined the lawsuit.
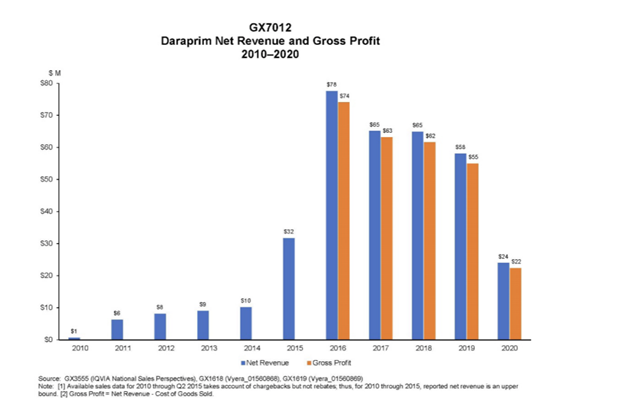
The lawsuit alleged Vyera engaged in unfair methods of competition, monopolization, and illegal trade restraint agreements to prevent entry of generic competition to its brand drug Daraprim. The complaint states that Vyera reaped $55 million to $74 million in annual profits from the sale of Daraprim at the newly inflated price. This is in stark contrast to Daraprim revenues for the years immediately preceding Vyera’s acquisition of Daraprim, which amounted to at most $10 million per year. The gross profit margin resulting from Vyera’s price increase is estimated to be as high as 98%.
To prevent generic entry, Vyera imposed restrictions in its distribution contracts, preventing the sale of Daraprim tablets to labs for bioequivalent testing.
Lifetime Ban and $65 Million Disgorgement
Judge Cote reviewed the FTC’s claims of violations of section 1 and 2 of the Sherman Act and the Attorneys General’s claims of individual state statute violations and found that they had satisfied their burden of proving both state and federal law violations by a preponderance of the evidence.
Regarding the Sherman Act Claims, Judge Cote found:
“Shkreli is liable for the violations of §§ 1 and 2 of the Sherman Act and the parallel violations of state law. Shkreli conceived of, implemented, maintained, and controlled Vyera’s anticompetitive and monopolistic scheme. His control continued after he stepped down as Vyera’s CEO and even after he entered federal prison. As the company’s largest shareholder, he freely changed its management and directed its policy.”
Judge Cote noted that Shkreli is a repeat actor, having previously employed similar tactics in acquiring drug rights to raise prices at a prior company Retrophin. In imposing the lifetime ban, Judge Cote noted:
“Shkreli’s anticompetitive conduct at the expense of the public health was flagrant and reckless. He is unrepentant. Barring him from the opportunity to repeat that conduct is nothing if not in the interest of justice.”
Judge Cote ordered disgorgement in the amount of $64.6 million as requested by the Attorneys General.
Vyera Health Care End Payor Settlement
Blue Cross Blue Shield of Minnesota (“BCBS Minnesota”), on behalf of a putative class of health care end payors, announced a settlement with Vyera Pharmaceuticals including Shkreli in December 2021. BCBS Minnesota’s class action lawsuit on behalf of end payors followed the claims of the FTC and Attorneys General complaints. A portion of the FTC settlement will be used to satisfy the BCBS Minnesota settlement.